Merck Covid Drug
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID-19 symptoms had about half the rate of hospitalization and death as patients who received a dummy pill. Were talking about a return to maybe normal life.
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID-19 symptoms had about half the rate of.

Merck covid drug. A pill to treat Covid-19. Merck said its new trial will study experimental drug molnupiravir for the prevention of COVID-19 among adults in the same household as someone diagnosed with symptomatic coronavirus infection. Mercks Little Brown Pill Could Transform the Fight Against Covid.
Merck and Ridgeback Biotherapeutics said Friday theyve developed a drug that reduces the risk of hospitalization or death by around 50 for patients with mild or moderate cases of Covid. Merck has said it would license the drug to generic drug manufacturers based in India making it available to people infected with COVID. The companies said the drug molnupiravir halved the.
The drug named molnupiravir reduces the risk of hospitalization or death by 50 in Covid-19 patients with mild and moderate symptoms compared with placebo Merck said. Shares posted their biggest gain in 12 years after the companys experimental pill slashed the risk of getting seriously ill or dying from Covid. Government 17 million doses of molnupiravir an oral antiviral treatment for COVID-19.
The study tracked 775 adults with mild-to-moderate COVID-19 who were considered higher risk for severe. Merck Co. Merck Says Research Shows Its COVID-19 Pill Works Against Variants.
In an early look at the data from a Phase 3 study the company said the drug lowered the risk of. Pfizer Inc and Merck Co Inc announced on Wednesday new trials of their experimental oral antiviral drugs for COVID-19 as the race to develop an easy-to-administer treatment for the potentially. Merck Co IncHandout via REUTERS.
Merck and closely held Ridgeback Biotherapeutics said Friday their oral drug molnupiravir reduced the risk of hospitalization or death by about 50 in patients with mild to moderate Covid-19. Laboratory studies show that Merck Cos MRKN experimental oral COVID-19 antiviral drug molnupiravir is likely to. If those promising preliminary results hold the new drug could help fill a.
Merck and its partner Ridgeback Biotherapeutics said early results showed patients who received the drug called molnupiravir within five days of COVID-19 symptoms had about half the rate of. Merck announced Friday it will ask the Food and Drug Administration to grant emergency use authorization for an anti-coronavirus drug that reduced hospitalizations by nearly 50 in clinical trials. At the interim analysis molnupiravir reduced the risk of hospitalization or death by approximately 50 Merck.
Merck announced encouraging results form a study of its COVID-19 antiviral drug molnupiravir. Merck announced that its new antiviral drug reduced the risk of hospitalization or death of COVID-19 patients by 50 in a late-stage trial. The antiviral drug molnupiravir still in clinical trials would give doctors an important new treatment and a.
Merck announced Wednesday that it has reached an approximately 12 billion deal to supply the US. Merck Antiviral Pills Clinical Trial Success Is Good News Fauci Says Health officials said the drug could provide an effective way to treat Covid-19 but stressed that. Laboratory studies show that Merck Cos experimental oral COVID-19 antiviral drug molnupiravir is likely to be effective against known variants of the coronavirus.
Oct 1 Reuters - Merck Co Incs MRKN experimental oral drug for COVID-19 molnupiravir reduced by around 50 the chance of hospitalization or death for. Mercks promising experimental Covid-19 drug raises hopes for pill to fight virus. Merck and Ridgeback say theyll apply as soon as possible to get their COVID-19 pill authorized.
Merck says its new pill reduces deaths by half in new coronavirus patients If cleared Mercks drug would be the first pill shown to treat COVID-19 a potentially major advance in efforts to fight. Merck said it would ask US regulators to authorise the first antiviral pill to treat Covid-19 after a late-stage clinical trial showed the drug cut the risk of hospitalisation or death in half.

Merck Mrk Molnupiravir Pill Could Change The Fight Against Covid Bloomberg











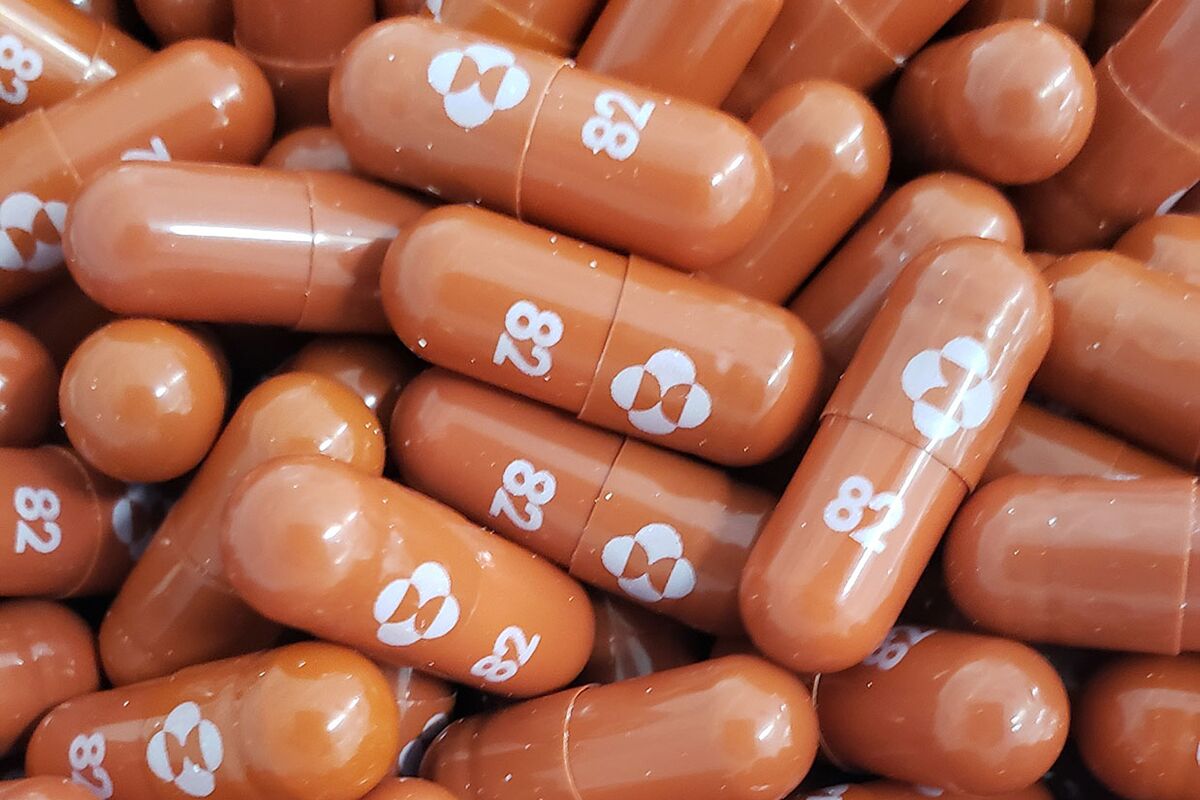
0 Response to "Merck Covid Drug"
Post a Comment